Element Science
Lifesaving wearable solutions addressing the leading causes of death and hospitalization due to heart disease
Overview:
The company was founded in 2011 by Uday Kumar, MD, a cardiac electrophysiologist by training, and founder of iRhythm technologies, a leading digital healthcare company focused on cardiac rhythm diagnosis. Element Science is developing a platform that will address the leading causes of death and hospitalization due to heart disease.
Technology and Pipeline:
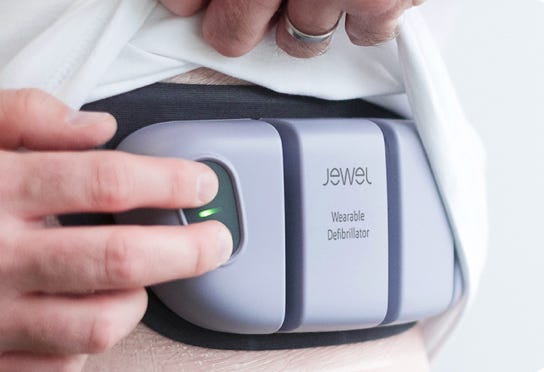
The first problem Element is trying to solve is sudden cardiac death (SCD). In the US, SCD claims 325,000 lives every year. The solution is a patch-based Wearable Cardioverter Defibrillator (WCD) that monitors a patient’s heart and protects the patient.
(Source: Element Science)
(Source: Element Science)
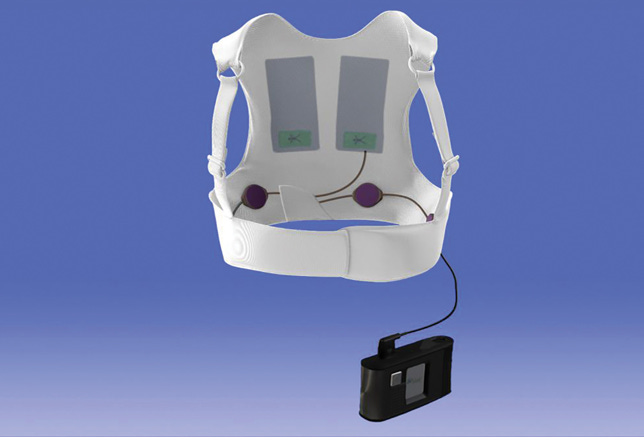
The product is comfortable and easy-to-use, while also being discreet and hassle-free. Currently the LifeVest is the only WCD to obtain FDA approval, which it received in 2001.
(Source: LifeVest)
(Source: LifeVest)
(Source: LifeVest)
Just by looking at the pictures you can tell which device you would rather wear. In addition, Element Science’s device is able to provide continuous monitoring throughout the day including while you shower and sleep. On top of that, the device comes with an app that keeps you connected to healthcare professionals 24/7.
There are many issues with the LifeVest as noted in the above chart. First, the vest only comes in two sizes which decreases the effectiveness for patients who do not fit the vest properly. In addition to being washed every couple of days, the patient is responsible for replacing the electrode belt, which results in patients being shocked incorrectly or inappropriately. Element Science’s WCD corrects this by having a one size fits all patch that is placed on the chest; this potentially not only ensures efficacy for all patients, but also reduces the odds a patient is shocked incorrectly due to the fact that there are less variables subject to human error. Element improves upon the user interface in the form of an app that vastly improves the user experience by eliminating any ambiguity in alerts that may leave patients confused or unsure. As a result, Element’s device is able to potentially better prevent SCD through increased patient adherence than the LifeVest.
There are a couple key takeaways from Dr. Kumar’s interview at the Society of Physician Entrepreneurs in San Francisco. First, they set out to focus on the user experience and the design of the WCD. The team wanted to see how patients interacted with WCD’s and what design would really move the needle economically for the patient. They found that patients care about not having anyone else know they are sick (discreet design), and patients don’t want to be reminded that they can die (LifeVest clunky and constant reminder; Jewel small and hidden under clothes, easy to forget about). These little takeaways early on drove the user experience and final design of the Jewel.
Clinical Data and Regulatory:
This device will have to receive FDA PMA approval given that it is a class III device. Class III devices are defined as those that support or sustain human life, are of substantial importance in preventing impairment of human health, or which present a potential, unreasonable risk of illness or injury. The PMA approval is the most stringent type of device marketing application by the FDA. Approval is based on determination by the FDA that the PMA contains sufficient valid scientific evidence to assure that the device is safe and effective for its intended use.
The VEST trial was conducted with the LifeVest
The Vest Prevention of Early Sudden Death Trial (VEST) is the first and only multi center randomized controlled trial to investigate the role and benefit of WCD. A total of 2,302 patients were included in the study mainly from the United States and Poland, and an intention-to-treat analysis did not demonstrate a difference in the primary endpoint of arrhythmic mortality (1.6% in the WCD arm vs. 2.4% in the control arm, P=0.18). Appropriate shocks were observed in 1.3% and inappropriate shocks in 0.6% patients; thus, a third of all shocks delivered were inappropriate. Another major issue with the available version of the LifeVest is the rate of compliance with daily use. Thirty percent of the participants stopped wearing the WCD within 1 month of randomization, 43% within in 2 months, and 80% before the end of the planned 90 day follow-up. The study not only failed to meet its primary endpoint, but also a third of the patients could not even wear the WCD.
(Source: LifeVest study)
This study presents WCD’s in a negative light, but I think it highlights how inadequate of a product the LifeVest is, not how WCD’s have no utility. It should also be noted that there are two potential cohorts for the WCD: those patients who don’t have an Automatic Implantable Cardioverter Defibrillator (AICD) and those patients who already have an AICD.
Market:
By 2018, when nearly 300,000 patients had used the LifeVest worldwide, sales were estimated to have increased to $700-$800 million. This is a significant market for a product that has so many inadequate features. If Element Science is able to execute on the Jewel, they should be able to provide superior value to patients over the LifeVest, thus taking market share from the incumbent.
(Source: Sales estimates)
As noted above, there are two cohorts that SCD patients should be divided into: those with an AICD and those without an AICD. There is an obvious need for patients without an AICD to have Element Science’s WCD; however, patients with an AICD represent a segment of the market where the current standard of care is already highly effective and eliminates any problems with patient adherence. With that being said, there is the potential for Element Science to show similar and/or greater efficacy as an AICD, but I will not hold my breath on that one. There is not much public information available in regard to the device, and I can’t find any clinical trials on clinicaltrials.gov.
Management:
Element Science is led by Uday Kumar, MD, who is the founder and CEO. Dr. Kumar also founded iRhythm Technologies and is apart of the Stanford Biodesign faculty. He serves as Entrepreneur-in-residence at Third Rock Ventures. Zubin Eapen, MD, was hired in late 2020 to serve as Chief Medical Officer. Dr. Eapen is a Duke trained cardiologist and also spent time with Anthem, CareMore, and HealthCore.
The Chairman of the board is Hank Kucheman, who is the former CEO of Boston Scientific. Neil Exter, partner at Third Rock, also serves on the board.
(Source: Our Team)
Financing:
Element Science raised a $12.5MM series A in 2014 from GV, Third Rock, and Prospect Ventures. In 2016, the company raised a $25MM series B led by GV and Third Rock. Recently, Element Science raised a massive $145MM series C round from GV, Cormorant Asset Management, Invus, Deerfield, Qiming Venture Partners USA, and Third Rock.
The latest raise will fund the completion of clinical studies and the launch of their first product. Given the size of this latest round, in addition to the crossover funds that participated, it would not be surprising to see Element go public sometime in 2H21 or early 2022.
(Source: Crunchbase)
Summary:
The founder of Element Science, Dr. Kumar, previously founded iRhythm Technologies. iRhythm launched the Zio, which is a wearable patch that is able to detect and diagnosis patients with irregular heart rhythms. The Zio can be worn for 14 days straight, even while exercising and showering, while the Holter monitor can only be worn for 24-48 hours. This technological advantage allows the Zio to capture data for the 51% of patients who have their first symptom-triggered arrhythmia after 48 hours (Source: Zio). I point this out because clearly Dr. Kumar has a unique insight into how to apply technology in a way that provides significantly more value for the patient than the current standard of care. There is no doubt there is one cohort where technology can provide significantly more value to the patient than the current standard of care (LifeVest), but the value to patients who already have an AICD remains uncertain. The difference between these two cohorts is what can make this a great product or a revolutionary product.
Element Science is rather sparse on the information they provide to the public and on their website, thus I was able to only conjure up as much information as I could. In any event, this seems like a really interesting company within the medtech space that has the potential to launch game-changing tech in an acute market.
Questions:
The LifeVest uses 4 dry sensing electrodes that are prone to skin motion or loss of contact; this necessitates compensatory algorithms to minimize false alarms and inappropriate therapy. The two programmable zones are customizable for detection time and shock therapy, which is not optimal and largely left to the physician. Is Element’s device able to achieve this optimal range of sensitivity/specificity and how do the achieve it? (Source: Heart Rhythm Paper)
Is it possible for the Jewel to have similar and/or greater efficacy than an Automatic Implantable Cardioverter Defillabrator?
What is the reimbursement process?
If anyone has greater information in regards to the technology behind Element Science as well as the clinical study, please DM me!
Additional links and articles: